

#CHECKMATE 577 TRIAL#
The CheckMate -577 trial will continue as planned to allow for future analysis of the secondary endpoint of overall survival (OS). The CheckMate-577 study found that patients who received Opdivo (nivolumab) after chemoradiation and esophagectomy experienced a doubling of the time until recurrence compared to patients who received a placebo.
#CHECKMATE 577 FULL#
The company will complete a full evaluation of the available CheckMate -577 data and work with investigators to share the results at an upcoming medical conference, as well as discuss them with health authorities. We plan to provide our data to health authorities worldwide with the goal of bringing Opdivo as an adjuvant therapy to these patients with high unmet need.”
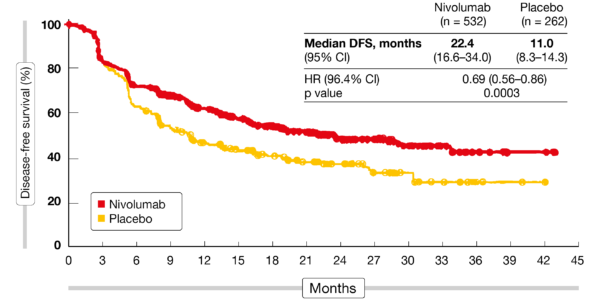

“The results from CheckMate -577 are immensely important for physicians and patients, and have the potential to establish Opdivo as a new standard of care. Waxman, M.D., development lead, Gastrointestinal Cancers, Bristol Myers Squibb. “Opdivo is the first and only therapy to improve disease-free survival, along with a manageable safety profile, for patients with esophageal or gastroesophageal junction cancer following neoadjuvant chemoradiation therapy and surgery,” said Ian M. At this event, it was presented the first data from CheckMate 577 (NCT02743494), a phase III, randomized, multicenter, double-blind trial evaluating the efficacy and safety of adjuvant nivolumab versus placebo in patients e sophageal or resected g astroesophageal j unction c ancer, whose specimen after trimodal therapy (neoadjuvant chemoradi. For the first time, we have a potential therapeutic option with nivolumab in the adjuvant setting for these patients.” “Medical oncologists have had limited to no treatment options to offer esophageal cancer patients who undergo neoadjuvant chemoradiation therapy followed by surgery and fail to demonstrate a complete pathological response. Sammons Cancer Center at Baylor University Medical Center. Kelly M.D., MBA, Director of the Charles A. “Approximately 50% of patients with esophageal or gastroesophageal junction cancer who undergo neoadjuvant chemoradiation therapy followed by tumor resection will have disease recurrence within four years, and among those who do not respond completely to neoadjuvant treatment, recurrence will occur sooner,” said Ronan J. This is the second tumor, in addition to melanoma, where Opdivo has demonstrated a benefit in the adjuvant setting. The safety profile of Opdivo was consistent with previously reported studies. Until those results are revealed, physicians should be hesitant about using them in these contexts, says Ko.In the trial, treatment with Opdivo following neoadjuvant chemoradiation therapy (CRT) and complete surgical resection demonstrated a statistically significant improvement in the primary endpoint of DFS compared to placebo in the all-randomized population. CheckMate-577 might reveal that the use of some of these novel agents, like immunotherapy, may at some point find their place in earlier-stage settings. Physicians need to figure out what to do with patients who have gone to surgery, but were found to have residual pathologic disease at the time of surgery. In the adjuvant setting, CheckMate-577 is looking at nivolumab (Opdivo) as an adjuvant treatment following chemoradiation and surgery for resectable esophageal and gastroesophageal junction cancers. However, a number of studies are looking at moving these targeted agents to first-line treatment as well as the perioperative setting.

Ko suggests to not automatically assume that these agents can and should be moved to earlier-line settings. As physicians eagerly anticipate the impact of a number of these drugs, an important take-home message is to be more circumspective. The greatest interest is in immune-oncology agents. Andrew Ko, MD, professor, Department of Medicine, University of California, San Francisco Helen Diller Comprehensive Cancer Center, discusses exciting data from the 2018 Gastrointestinal Cancers Symposium.


 0 kommentar(er)
0 kommentar(er)
